The pentose phosphate pathway (PPP) is also known as the hexose monophosphate (HMP) shunt. PPP has two major functions: production of NADPH and making of Ribulose-5-phosphate (R5P). R5P is needed for the synthesis of DNA, RNA, ATP, NAD, FAD, CoA, etc… NADPH is used 1) in the synthesis of fatty acids, cholesterol, and hormones, and 2) to prevent and reverse oxidative damage through the maintenance of a supply of reduced gluthathione. The PPP can be tuned to either NADPH production or R5P production.
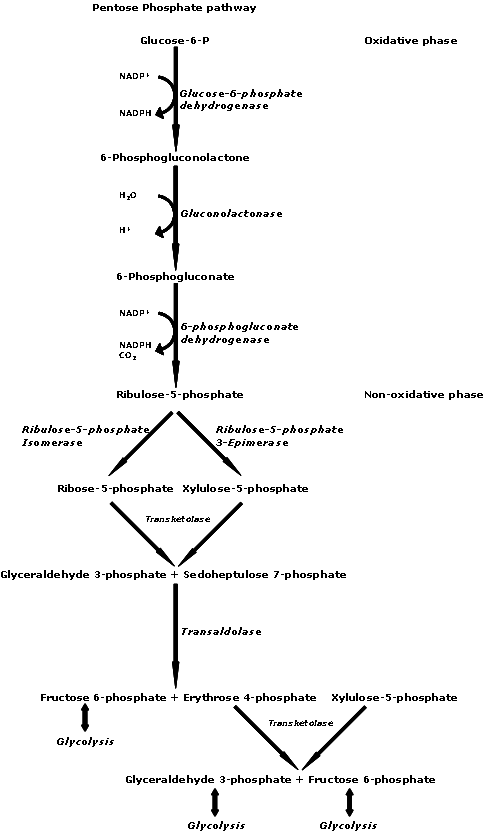
The non-oxidative reactions of PPP have the net reaction: 3 ribulose-5-phosphate → 1 ribose-5-phosphate + 2 xylulose-5-phosphate → 2 fructose-6-phosphate + glyceraldehyde-3-phosphate. These are all reversible reactions that produce an equilibriated pool of sugars for biosynthesis; F6P and GAP (aka G3P) can feed directly into glycolysis. These interconversions, shown below, are mainly accomplished by transketolase and transaldolase. The “big picture” is 5C + 5C -> 7C + 3C.

Transketolases transfer two-carbon fragments with Thiamine Pyrophosphate (TPP) as a co-enzyme and transaldolases transfer three-carbon fragments using a Schiff base intermediate. Mechanistically, the recipient of the 2C or 3C fragment is always the aldose. C=O acts as the electrophile.
In the oxidative PPP reactions, two molecules of NADP+ are reduced to NADPH from the conversion of G6P to R5P. These reactions are irreversible. The rate-limiting enzyme is glucose-6-phosphate dehydrogenase (G6PD), which converts G6P to 6-phosphogluconate. A dehydrogenase is an enzyme that catalyzes the removal of hydrogen atoms from its substrate. G6PD is induced by insulin, since the abundance of sugar entering the cell under high insulin conditions will be shunted into fuel utilization pathways (glycolysis and aerobic respiraiton) as well as fuel storage pathways (fatty acid synthesis, glycogenesis, and the PPP). This shunt is inhibited by its product, NADPH, and activated by one of its reactants, NADP+.

Oxidative phase of pentose phosphate pathway.
Glucose-6-phosphate (1), 6-phosphoglucono-δ-lactone (2), 6-phosphogluconate (3), ribulose 5-phosphate (4)