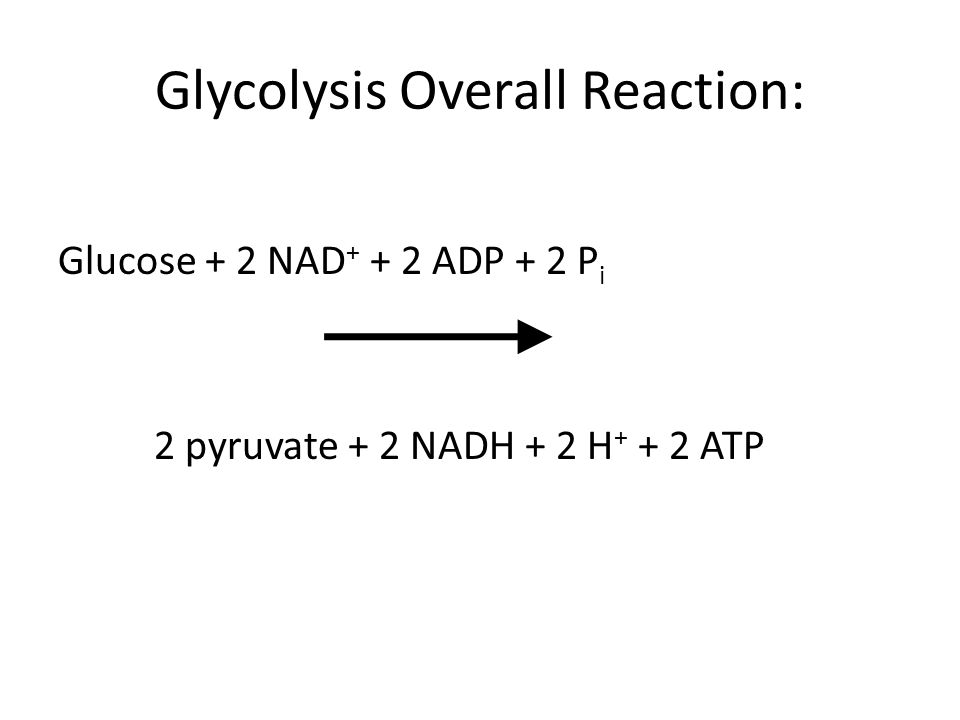
Glycolysis is an evolutionarily old pathway that is present in all cells. Life could not exist without the ability to carry out glycolysis. Glycolysis occurs in the cytoplasm of cells, converting one glucose molecule to 2 molecules of pyruvate and a small amount of energy in the form of 2 ATP’s and one NADH. If mitochondria and oxygen are present in the cell, the NADH may be redeemed for ATPs further downstream. Nevertheless, glycolysis occurs anaerobically.
Glycolysis involves 10 distinct steps, with 10 different enzymes, and can be broken into 2 phases, the preparatory phase (first 5 steps) and the pay-off phase. The preparatory phase can also be known as the investment phase, since ATP is consumed by these reactions. Shown below is the full glycolysis pathway. Before glycolysis can occur, however, glucose must be present in the cytoplasm of the cell. Glucose can enter the cell through either facilitated diffusion or active transport.

1. Hexokinase phorylates glucose into glucose-6-phosphate. Hexokinase is the first enzyme of glycolsis. A kinase attaches a phosphate group from ATP to its substrate. By converting glucose into glucose-6-phospate (G6P), this step traps glucose in the cell, since the transmebrane transporters of glucose are only specific for glucose. Another effect of this conversion is the maintenance of a low glucose concentration in the cytoplasm, which ensures the continued transport of glucose into the cell. Hexokinase is inhibited by G6P, so a high concentration of G6P will prevent the production of more. While hexokinase is the enzyme responsible for this step in most tissues, liver hepatocytes and pancreatic β-islet cells uses glucokinase instead. Glucokinase is also known as Hexokinase 4. This reaction is considered irreversible.
2. Phosphoglucose Isomerase (PGI) rearranges G6P into its isomer Fructose-6-phosphate (F6P). PGI is also known as Glucose-6-phosphate isomerase. An isomerase converts a specified compound to an isomer. The Glu active site of the enzyme functions as first a general base, then a general acid. An enediol intermediete is involved. This reaction is freely reversible under normal cell conditions, but is driven forward by a low concentration of F6P. F6P is readily consumed by the next reaction.
3. Phosphofructokinase-1 (PFK-1) phosphorylates F6P into Fructose 1,6-bisphosphate (F1,6BP). PFK-1 is subject to complex allosteric regulation and is indeed the main control point in glycolysis. This step is essentially irreversible with a ΔG ~ 22 kJ/mol under typical reaction conditions. ATP is consumed in this step. Therefore, it’s the committed step in the glycolysis pathway. You may ask, why isn’t the hexokinase step the committed step in the pathway? The answer is that G6P isn’t only used for glycolysis; G6P is the starting material for the Pentose Phosphate Pathway and can also be converted to glucose-1-phosphate for glycogenesis. PFK-1 is inhibited by ATP and citrate and is activated by AMP. This makes intuitive sense because high ATP concentration suggests a cell has sufficient energy and high citrate (a TCA cycle intermediete) also suggests a cell has sufficient energy, since TCA is producing much more ATP. AMP suggests the cell needs energy. In hepatocytes, insulin stimulates PFK-1 and glucagon inhibits it through an indirect mechanism involving PFK-2 and fructose 2,6-bisphosphate (F2,6BP). Insulin, which is released after a carbo-rich meal, activates PFK-2 to convert a small amount of F6P to F2,6BP, which activates PFK-1. Glucagon, on the other hand, which is released when blood sugar is low (“fasting”), inhibites PFK-2, lowering F2,6BP and thereby inhibiting PFK-1.
4. Aldolase cleaves F1,6BP into two 3-carbon (triose) sugars, dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP). There are 2 classes of aldolases, class I (animals and plants) and class II (fungi and bacteria). Class I aldolases form a protonated Schiff Base intermediete and link an active site lysince with C2 (covalent catalysis). Class II aldolases use divalent cations (Zn2+ or Mg2+). Thus, the C2 carbonyl carbon plays an important role in C3-C4 breakage.
This reaction is freely reversible under normal cell conditions.

5. Triosphosphate isomerase (TIM aka TPI) converts DHAP to its isomer GAP. So one molecule of F1,6BP yields 2 molecules of GAP. This is the last step of the preparation or investment phase.
This reaction is freely reversible under normal cell conditions.
6. This is the beginning of the pay-off phase, where 2 ATP’s are generated per GAP molecule, leading to a total gain of 4 ATP’s per glucose molecule. Since 2 ATP’s are invested to get this far, the net gain is 2 ATP’s per glucose molecule. Glyceraldehyde phosphate dehydrogenase (GAPDH) converts GAP into a “high-energy” phosphodonor, 1,3-bisphosphoglycerate (1,3-PG or 1,3-BPG), using oxidation of the aldehyde to drive the reaction. The phosphate comes from free floating Pi in the cytoplasm. The hydrogen lost is used to reduce a molecule of NAD+ to NADH + H+.
7. Phosphoglycerate kinase catalyzes the transfer of a phosphate from 1,3-PG to ADP, generating 3-phosphoglycerate (3-PG) and ATP. This is a substrate-level phosphorylation step, which is where ATP or GTP is formed by the direct transfer of a phosphoryl group to ADP or GDP. This is also the break-even point of glycolysis; 2 ATPs have been invested and 2 have been generated.
This reaction is freely reversible under normal cell conditions
8. Phosphoglycerate mutase isomerizes 3-PG into 2-phosphoglycerate (2-PG).
This reaction is freely reversible under normal cell conditions
9. Enolase converts 2-PG into phosphoenolpyruvate (PEP).
This reaction is freely reversible under normal cell conditions
10. Pyruvate kinase performs substrate-level phosphorylation to convert PEP and ADP to pyruvate and ATP. Pyruvate kinase is activated by F1,6BP from the PFK-1 reaction, which is a feed-forward activation.
This reaction is irreversible under normal cell conditions.