Formation of Acetyl-CoA – Pyruvate Dehydrogenase Complex
There are many different starting molecules from which to form acetyl-CoA. Here, we’ll go over the formation of acetyl-CoA from fatty acids, ketones, carbohydrates, amino acids, and alcohol. Acetyl-CoA is important because it can be used to generate large amounts of energy in the TCA cycle and subseuquent oxidative phosphorylation.

One of the most common ways of generating Acetyl-CoA under normal conditions is from carbohydrates. Specifically, the pyruvate produced by glycolysis can be converted to acetyl-CoA by a multienzyme complex known as the pyruvate dehydrogenase complex (PDH). This complex is located in the mitochondrial matrix and allows acetyl-CoA is produces to feed into the TCA cycle. Pyruvate is transported by the pyruvate transporter into the matrix. Although the overall reaction for PDH may look straightforward,

the mechanism actually involves multiple steps and 5 intermedietes. These are TPP, lipoic acid, FAD, CoA-SH, and NAD+. Its mechanism is a classic example of “substrate channeling.” In the first step, pyruvate is decarboxylated with the help of TPP and the enzyme pyruvate dehydrogenase. TPP is special because it has an acidic carbon. In this step, one CO2 from pyruvate is released and the TPP carbanion ends up covalently bonding to pyruvate. Next, in the second step, lipoamide oxidizes the molecule from step 1, allowing TPP to leave and instead binding the 2-carbon molecule to lipoamide. Mechanistically, the carbanion attacks one lipoamide sulfur, with the disulfide group in lipoic acid acting as an oxidizing agent. The reaction is catalyzed by the enzyme dihydrolipoyl transacetylase. In the third step, dihydrolipoyl transacetylase also catalyzes the CoA-SH addition to the two-carbon molecule, with the sulfur from CoA-SH attacking the sulfur-bound carbon. Note coenzyme A (CoA) is written as CoA-SH because CoA is a thiol. This forms acetyl-CoA but does not regenerate lipoamide, instead creating dihydrolipoamide. Flavin adenine dinucleotide (FAD) is used as a coenzyme to reoxidize lipoic acid in the fourth step, resulting in FAD being reduced to FADH2. In the fifth and final step, FADH2 transfers its electrons to NAD+, resulting in reformation of FAD and formation of NADH.
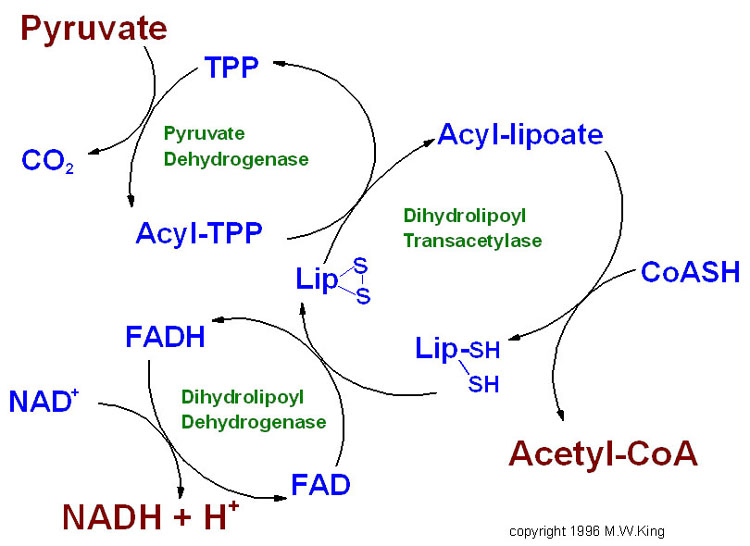
One of the coolest things about the PDH complex is the lipoamide arm, shown below. During the course of the reaction, the arm vists 3 different active sites within the PDH complex. In step 2, the arm inserts into the “E1” active side to pick up the acetyl group from TPP. In step 3, the arm visits the “E2” active site, where the acetyl group is transferred to coenzyme A. Acetyl-CoA is released. In step 4, the arm visits the “E3” active side to get re-oxidized for the next catalytic cycle. Step 5 also occurs in during this arm position.
Fatty acid oxidation (β-oxidation) can also generate acetyl-CoA. This process is discussed more in depth in the fatty acids section. Basically, fatty acids in the cytosol can be activated by forming a thioester bond between their carboxyl groups and CoA-SH. This activated fatty acyl-CoA cannot cross the inner mitochondrial membrane, but is transported accross by the carnitine shuttle. Once the acyl-CoA is in the matrix, β-oxidation generates acetyl-CoA.
Certain amino acids can loose their amino group via transamination to form ketone bodies with their carbon skeletons. Ketones can be turned into acetyl-CoA. Alcohol, consumed in moderate amounts, can be converted into acetyl-CoA by alcohol dehydrogenase and acetaldehyde dehydrogenase.